All of us know that H2O is the chemical formula of water. But how many of us have wondered why we write H2O instead of something else? What makes this particular alpha-numeric combination the formula of H2O?
If you have similar questions in your mind, then you have come to the right place. The answer to all these questions lies in the valency chart or what we popularly known as valency. But before we further dive into the valency chart, let us first understand what valency is.
What is Valency?
The valency measures the combining capacity of molecules or atoms. Hence, it refers to the atom’s capacity of one element to combine and react with a specific number of atoms of another element. This can further be explained in the following way:
An atom contains electrons arranged in various shells or orbitals, which are characterised as K, L, M, and so on. Valence electrons are the ones that are present in the last or outermost orbit. And during any chemical reaction, these valence electrons actively work as the outermost orbit contains more energy than the electrons in the inner orbits.
As per the theory of Bohr-bury, an atom’s outermost shell can contain a maximum of eight electrons. So, when an atom’s outermost orbit is filled with eight electrons, that particular element cannot engage in any chemical activity.
The combining capacity of that element becomes zero to negligible. Hence, noble gases are the least reactive elements in the periodic table as their outermost shell is filled with electrons. However, other elements’ reactivity depends on their capability to achieve noble gas configuration. This also helps in determining the atom’s valency.
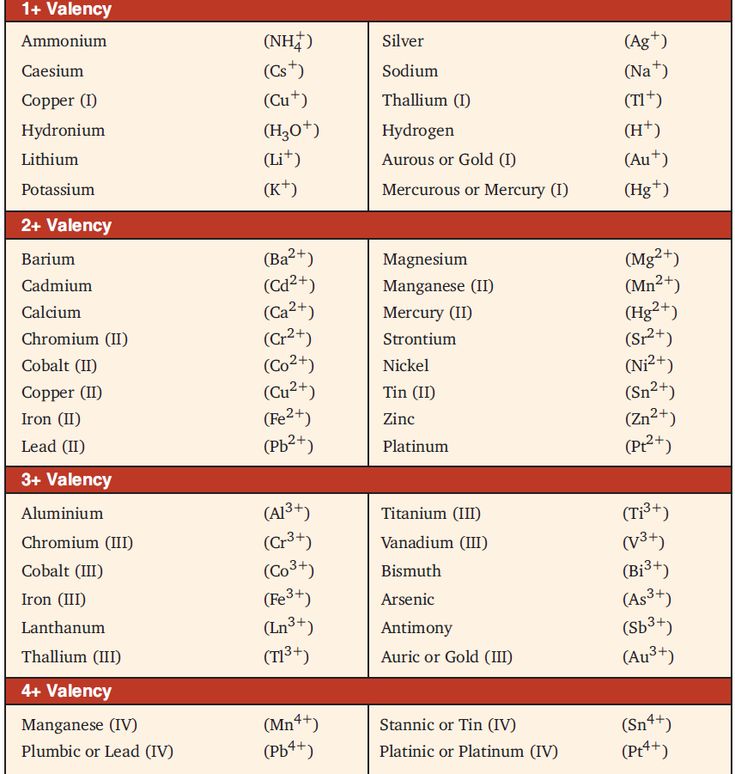
How To Determine the Valency of An Element
There are three easy ways of determining the valency of an element. These are as follows,
- The Octet Rule
According to the octet rule, the atoms have a tendency of keeping eight electrons in the outermost shell either by losing or by gaining electrons irrespective of the form of the compound. We know that the outermost shell of an atom can contain not more than eight electrons. An atom gains stability by keeping eight atoms in its outermost shell.
Therefore, an atom can lose up to four electrons from its outermost shell. An atom gains positive valency when it donates the free electrons. Similarly, an atom can gain or accept electrons when its outermost shell has four to seven electrons. In that case, the valency can be determined by subtracting the electrons’ quantity from a total of eight electrons.
- The Periodic Table
This is a simple method of determining the valency of an element. For instance, all metals present in the first column have a valency of 1. Likewise, all elements in column 17 have -1 valency. Column 18 presents the noble gases, which are inert elements and have 0 valencies. However, you can also tonic some exceptions as certain elements have multiple active shells, such as gold, iron, and copper.
This exception is noticed among transitional metals and other heavier elements, actinides, and lanthanides.
- The Chemical Formulae
This method is somewhat similar to the octet rule. You can determine the valency of the transitional elements by observing how it unites with other elements of known valency. In such cases, the octet rule can be followed as radicals and elements combine to keep eight elements in the outermost shell and stable.
We can take the example of sodium chloride (NaCl) to understand this point further. The valency of Chlorine is -1, and that of sodium is 1. Hence, both Chlorine and sodium need to lose and gain one electron each to have a stable outermost shell. Thus Chlorine accepts the electron donated by sodium. This helps in determining the valency.
Tips for Remembering Valency
Here’s how you can remember the valency chart with ease:
- Remember them in groups
When you read through a periodic table, you will notice that the valencies are written on the top of the atom. Hence, you can group the elements to remember their valances. For instance, elements in group 1 have 1 valency, elements in group 2 have 2 valencies. All the transition elements from groups 3-12 have positive valances. Whereas groups 18, 14, and 13 have variable charges. Lastly, groups 15 to 17 have negative valences.
- Colour-code them
Print out a periodic table and colour code the elements with the same valency. For instance, colour code the elements red with 1 valency. Similarly, use different colours of different elements sharing the same valency number. Colour coding is a fun way of remembering the valency of elements, and it provides you with the opportunity to engage with the periodic table and memorise it repeatedly.
- Memorise the atomic number
By now, you know that the outermost shell cannot contain more than 8 electrons. Hence, when you remember the atomic number of an element, you can easily determine the number of electrons it would contain in its outermost shell. Now depending on the electrons in the outermost shell, the atom will gain or lose electrons. This will help you determine as well as remember the valency of the element.
- Quiz yourself
A great way of remembering the valency chart is by quizzing yourself whenever possible. Ask yourself the valency of certain elements throughout the day. When you keep remembering what you have learned before and challenge yourself, it would be easier to learn the valency chart. For example, ask yourself the valency of titanium when you practice yoga to see whether you remember it correctly.
Wrapping Up
As you practice the valency chart, you will become more familiar with it. Moreover, memorising the atomic number of the elements would also help greatly. However, ensure that you understand the concept of valency. This would help you remember the chart naturally instead and prevent rote learning.
Read Also
Introducing Kids To Graphic Designing: Unleash Their Inner Graphic Designer